Why Aren’t We Tapping into the FDA’s ‘Back Catalog’?
There is enormous potential for repurposing well-known drugs that FDA has deemed safe.
Sen. Ron Johnson (R-Wis.) led a roundtable discussion in Washington on Monday, February 26, 2024, titled “Federal Health Agencies and the COVID Cartel: What Are They Hiding?”
Dr. Pierre Kory joined Mr. Johnson and a panel of experts to talk about how federal health agencies, Big Pharma, legacy media, and Big Tech engaged in censorship, collusion, and coverups.
Here is the text of Dr. Kory’s presentation:
Since its founding, the Food and Drug Administration (FDA) has approved approximately 32,000 medicines for practically every condition known to medical science. This massive library of treatments is a gift to humanity.
But like all libraries, it also contains secrets. We are only now beginning to understand that many long-established drugs have multiple mechanisms and can be used to effectively treat diseases with either similar or different pathophysiologies. Further, the longer they have been in use the more well-known is their safety profile.
Thus, there is enormous potential for repurposing this massive ‘back catalog’ of well-known drugs that FDA has deemed safe. So why on earth aren’t we systematically testing them for potential new uses?
The ugly truth is… it’s not profitable to do so. Big Pharma makes money on complicated new drugs and it effectively controls numerous levers of power. Nearly half of FDA’s budget is bankrolled by the drug industry, and its tentacles are deep in media, academia, medicine, and other regulatory agencies like the NIH. For decades it has waged war on safe, effective, off-patent treatments for numerous diseases, none more damaging than its war on Vitamin D.
Big Pharma’s main tactic is valuing randomized, controlled trials as the infallible ‘gold standard,’ while dismissing positive results of other kinds of studies, such as smaller RCTs and observational studies. The reality is that large randomized controlled trials cannot overcome the bias of their funders such that the only thing “controlled” about them is their results — as we saw numerous times in the pandemic.
Meanwhile, observational studies, which are far simpler and cheaper to perform, can produce results just as accurate as RCTs. A definitive 2014 Cochrane meta-analysis compared 10,000 RCTs and observational studies and concluded, “on average, there is little evidence for significant effect estimate differences between observational studies and RCTs.”
This cynical manipulation of evidence-based medicine isn’t a secret. As far back as 2009, Dr. Marcia Angell, the longtime editor of The New England Journal of Medicine, issued this prescient warning:
“It is simply no longer possible to believe much of the clinical research that is published, or to rely on the judgment of trusted physicians or authoritative medical guidelines… No one knows the total amount of money provided by drug companies to physicians, but I estimate from the annual reports of the top 9 U.S.-based drug companies that it comes to tens of billions of dollars a year in North America alone.”
This creeping, decades-long war on off-patent drugs went into overdrive during the pandemic, when Big Pharma turned its guns against early treatments for COVID. Using their control over the high-impact medical journals, they consistently published studies designed to show pre-determined negative results. With the size of these studies, and the control over the journals, they managed to convince the world of a lack of efficacy using only a handful of studies despite hundreds of others concluding efficacy.
Further, these studies’ numerous, fatal flaws were ignored. For example, to prove a medicine didn’t work, they used the lowest doses for the shortest courses while enrolling patients as late as possible while including only the mildest cases. They took the exact opposite tack with their favored trials to declare that patented medicines like Paxlovid worked.
What’s more, when they did not get the result they wanted, in several instances they were forced to change the original endpoint, a supposed “never-event” in research science. Despite these manipulations, each of these study’s publications launched massive negative PR campaigns and recommendations from health agencies against their use.
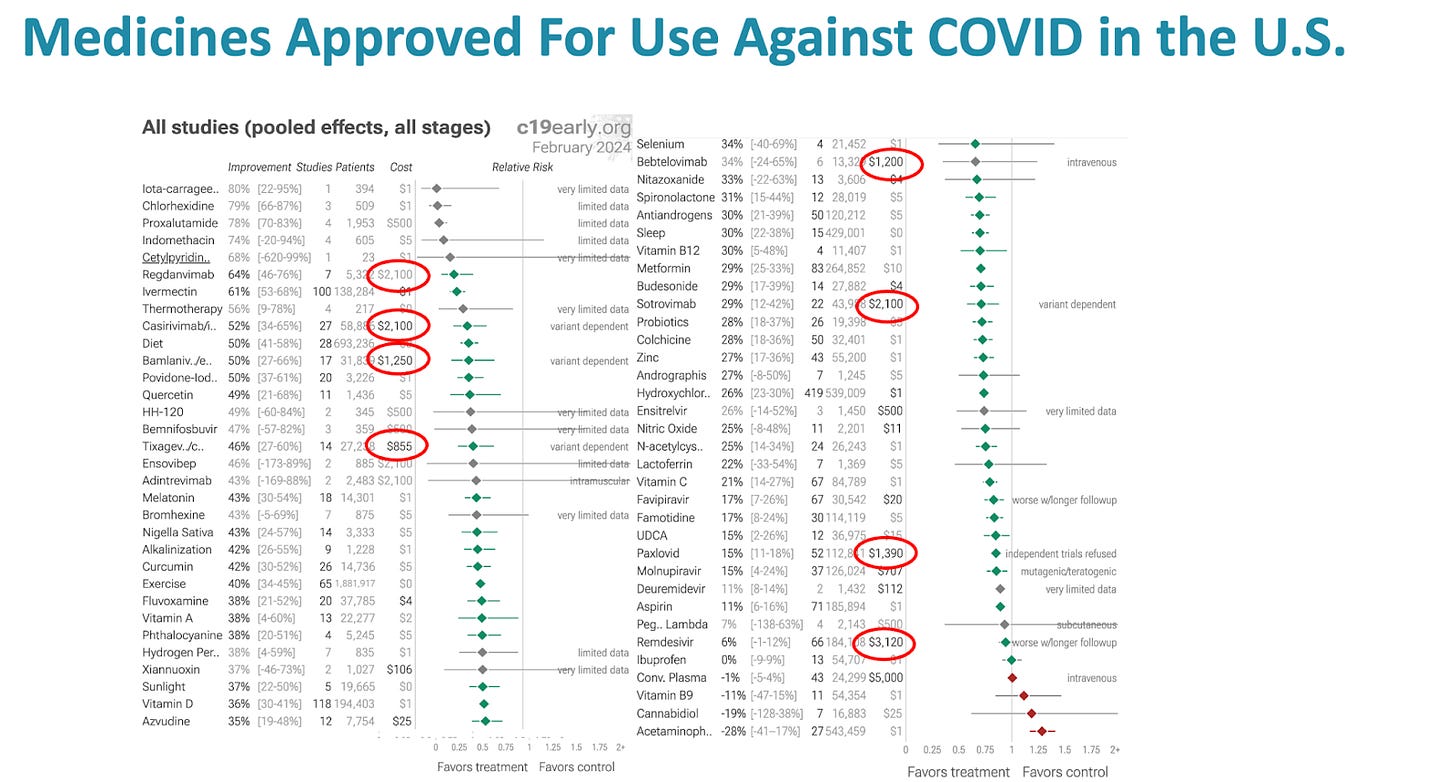
As of today, February 23, 2024, there have been 420 controlled trials studying HCQ including over half a million patients. With ivermectin, there have been 100 controlled trials with over 125,000 patients. Summary analyses of these evidence bases all show large magnitude, statistically significant benefits in all important outcomes.
Yet, in this country, IVM is considered an ineffective horse dewormer and HCQ the drug of fringe, quack, right-wing, anti-vaxxers.
So what’s the way forward from here? We need to create a framework to test off-patent and off-label drugs and model their clinical benefits and cost savings. A public-private partnership of diverse, independent and unconflicted stakeholders accountable to Congress could conduct sustained, independent, systematic studies of repurposed drugs to complement FDA review, clinical practice, and the role of natural immunity in health. It won’t be easy, but if physicians, healthcare leaders, and politicians unite behind this call to action, we can push the system towards greater accountability and help more people in the process.






For people with chronic illness that isn’t recognized (e.g. me/cfs/fm; autoimmune), repurposed drugs are all we’ve got. We’re already, quite literally, ridiculed when we walk into a doc’s office or ED. When it’s deigned that we get any treatment at all, it’s usually repurposed, off-patent anti-seizure medicine or a newer and more expensive reformulation that’s less effective. Autoimmune is often treated with repurposed antimalarials and corticosteroids.
When the anti-ivermectin hysteria began I was concerned we’d lose access to the repurposed drugs we’re allowed to have. If CA bill 2098 hadn’t been squashed in court it would’ve set a horrible precedent for those of us whose conditioners have no consensus on dx or tx. We were very close to losing what little help we have.
Because then all these clot shots would not be needed………I called “FOUL” very early when HCQ was verboten… I took it for 60 days with Cipro in 1996 for Lyme Disease.