Towards Robust Pharmacovigilance Surveillance Systems
Current systems' data is ‘meaningless’, and doing summary statistics on the VAERS dataset is ‘dumpster diving’.
FLCCC Director of Scientific Research, Matthew Halma, points out the need for effective pharmacovigilance
There has been an obvious failure of the present pharmacovigilance to respond to the notable safety concerns with the products known as Covid-19 vaccines. Simultaneously, the systems are supposedly so robust that they are capable of noticing a safety signal, yet when it comes to their actual use, their data is ‘meaningless’, and doing summary statistics on the VAERS dataset is ‘dumpster diving’.
Essentially, VAERS is supposed to be the last line of defense between harmful pharmaceutical products and the public, yet the defenders of this as a robust pharmacovigilance system are also quick to point out that its data are unusable to determine safety concerns. If this is confusing to you, it may simply be that you are not smart enough to understand what the public health establishment (with their illustrious PhD’s and MPH’s) has learned throughout the course of their studies.
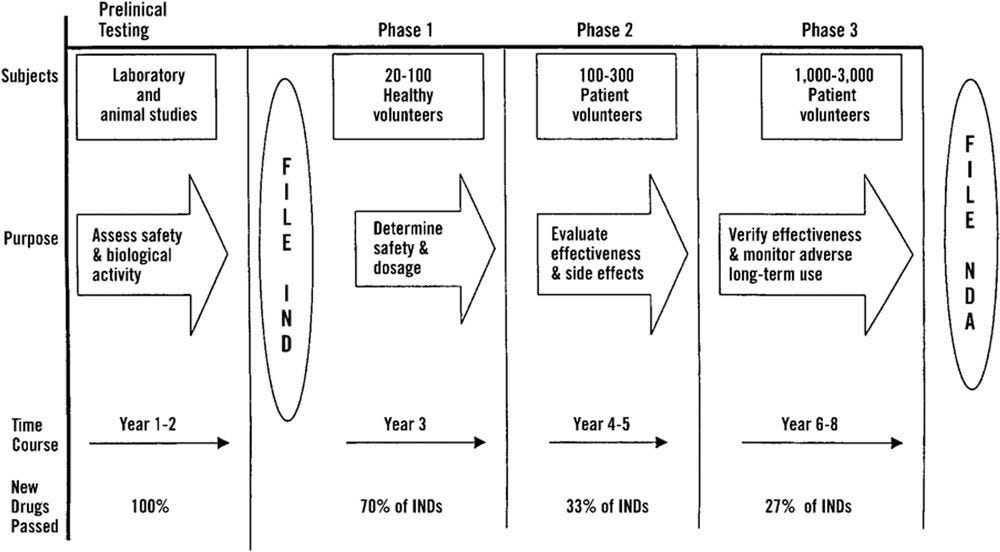
This obfuscation belies a clearer and less contorted truth, that there is no functioning pharmacovigilance system in the USA and throughout the world. The US FDA is more permissive and faster to approve drugs than its European counterpart, the European Medicines Agency (EMA) (1). Generally, there is agreement in regulatory decisions, but admittedly a fair bit of deviation; of the 115 new active substances approved by the EMA or FDA in the time period 2014-2016, 82/115 (71%) were approved by both agencies, 24 were approved solely by the US FDA, and 9 were approved only by the European EMA (Figure 1).
It has been pointed out previously that unless a pharmacovigilance system is sufficient to assign causality, then it cannot be considered a functioning system (2,3). It may be that the lack of functioning of pharmacovigilance systems are a feature, not a bug.
Upton Sinclair once said: “It is difficult to get a man to understand something, when his salary depends on his not understanding it”.
Conflicts of interest are rife within the US FDA (4–6),and this influences voting behavior (7). In other news, water is wet.
How do we restore true pharmacovigilance? A new article published in the journal Open Health, “Towards robust pharmacovigilance surveillance systems’, outlines the necessary changes to pharmacovigilance (or more likely, principles of a new pharmacovigilance system) (8).
Firstly, there needs to be a culture of reporting adverse events related to medical treatments, instead of a culture of persecuting doctors who submit reports.
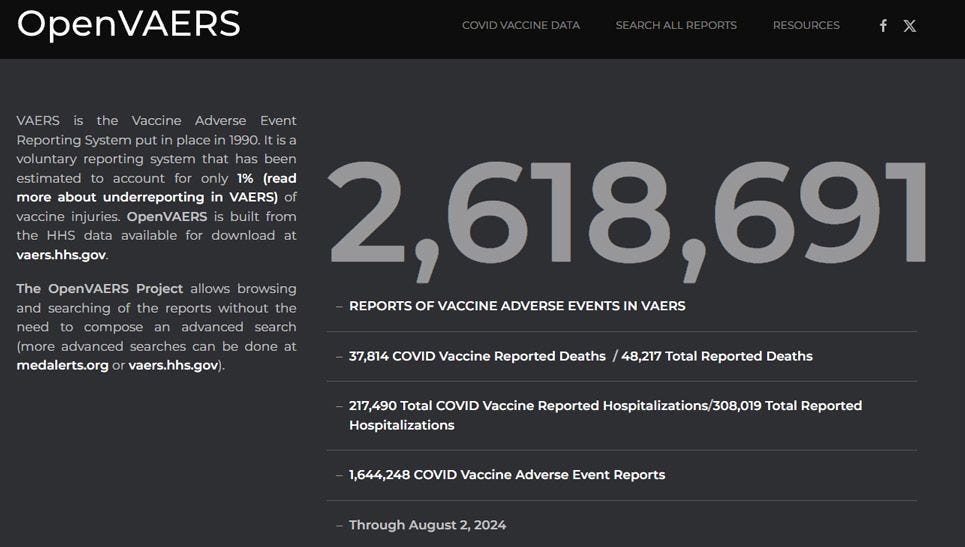
Secondly, the data must be accessible, not warehoused in a byzantine system. The initiative OpenVAERS has been very helpful at making vaccine adverse event counts more accessible. Most of you have probably seen this graph of vaccine-related adverse events by year (Figure 2). This was made possible due to the work of the dedicated team at OpenVAERS to make the data available to the general public.
Thirdly, the adverse event reports have to mean something. It means nothing if your ‘check engine’ light is flashing if you just ignore it or try to cover or disable the light. Safety signals need to be thoroughly investigated and if the drug is unsafe (which is certainly the case for the Covid-19 biological products), regulators need to step up and take the drug off the market.
Fourthly, adverse event reporting systems can benefit from harmonization with others. Many aware doctors and journalists were made aware of the safety issues with Covid-19 mRNA ‘vaccines’ from the data in Israel (9). Had we relied solely on data from the USA, we would not have been aware of the longer-term effects. It is said that dumb people keep making the same mistakes, smart people learn from their mistakes, and wise people learn from the mistakes of others. In this dichotomy, our public health establishment is decidedly in the first category.
Overall, this work provides a framework for future pharmacovigilance systems in a health system which truly values public health, rather than seeing the public as potential customers first and foremost.




“How does a vaccine that turns our cells into viral antigens confer immunity and not disease?
“Those who make us believe in absurdity can make us commit atrocities.” Voltair
In an attempt to better understand the vaccine safety tracking problem, the U.S. Department of Health and Human Services gave a $1 million grant to Harvard Medical School in 2006 (ESP:VAERS). This project tracked VAERS reporting at Harvard Pilgrim Healthcare for three years in an effort to “create a generalizable system to facilitate detection and clinician reporting of vaccine adverse events, in order to improve the safety of national vaccination programs.” At the end of the study the researchers determined that:
Adverse events from drugs and vaccines are common, but underreported. […] Likewise, fewer than 1% of vaccine adverse events are reported. Low reporting rates preclude or slow the
identification of ‘problem’ drugs and vaccines that endanger public health […and that…] new surveillance methods for drug and vaccine adverse events are needed.
So, what did the CDC do after the new system was ready to be implemented? Nothing. This is not new. (And advances in AI should make auto-reporting even easier. But of course the more issues you find…)
https://digital.ahrq.gov/ahrq-funded-projects/electronic-support-public-health-vaccine-adverse-event-reporting-system